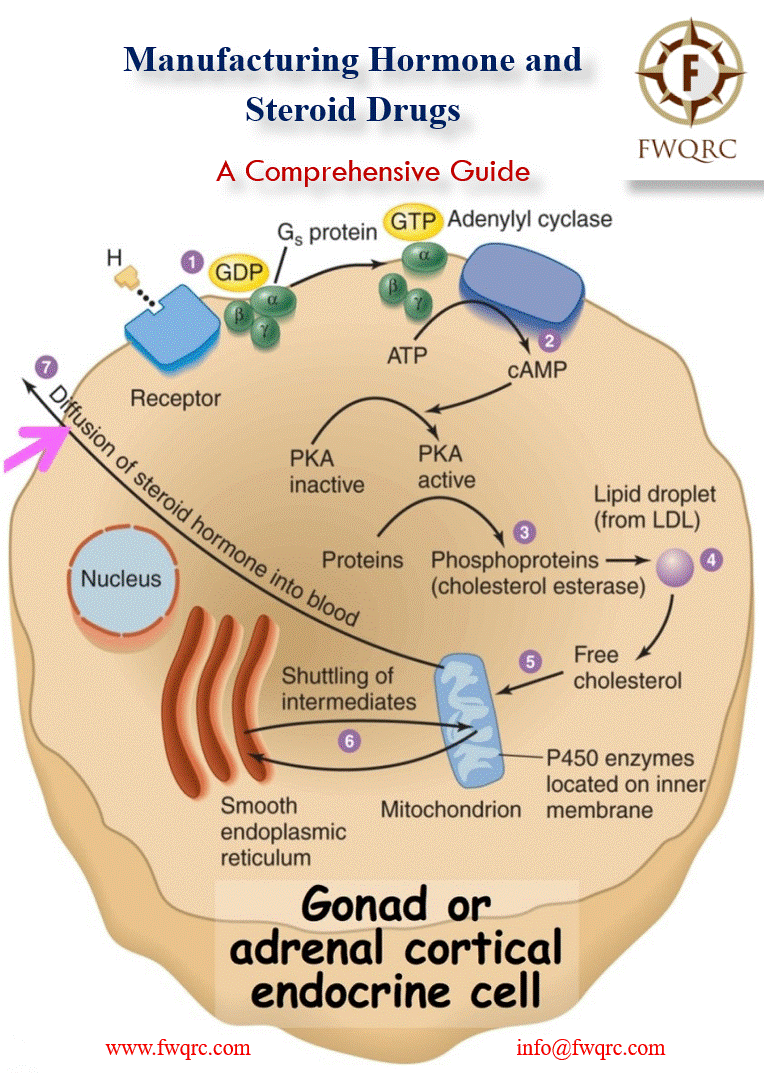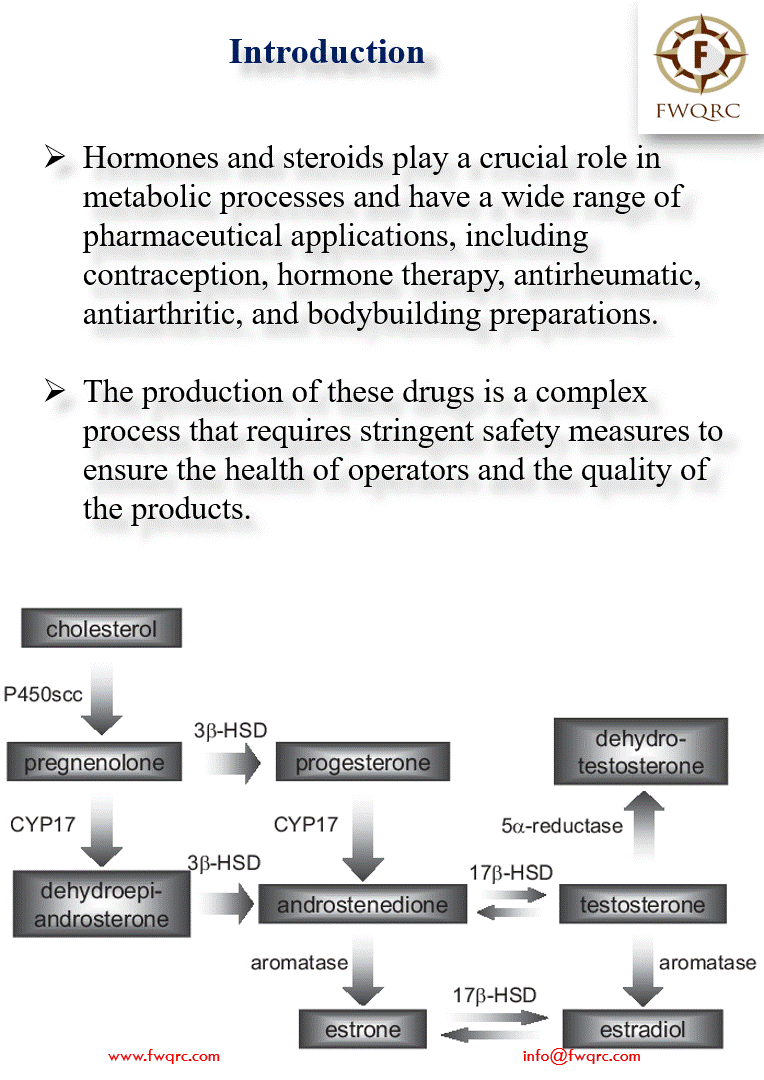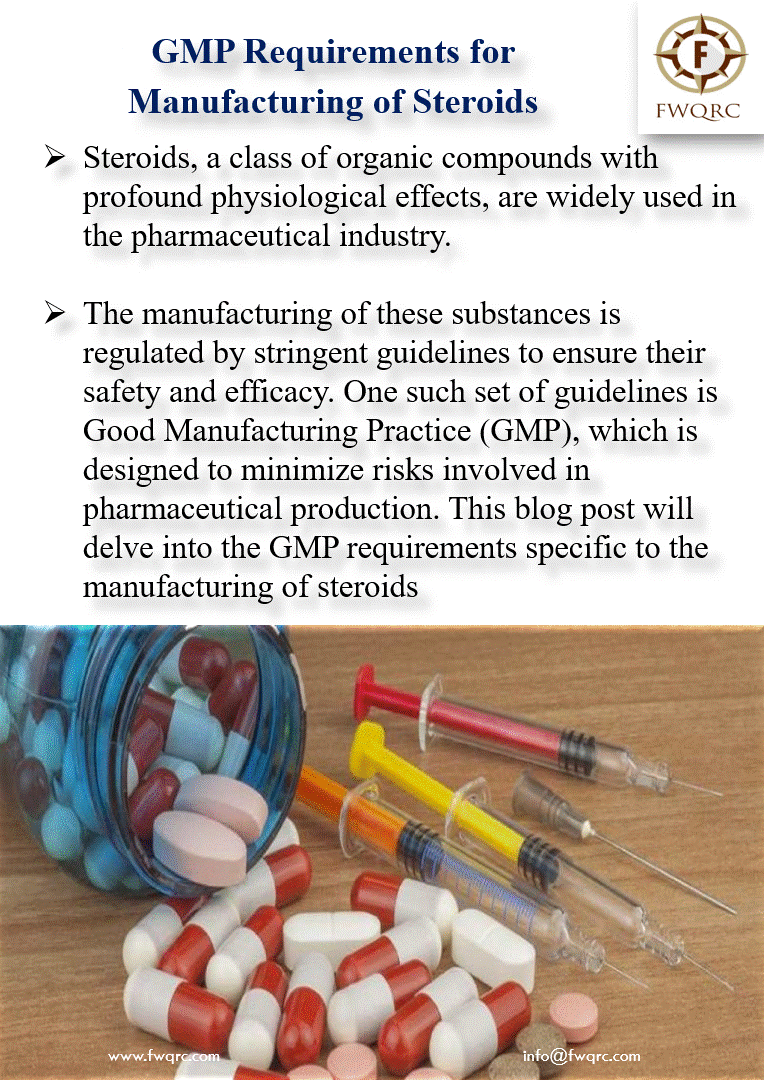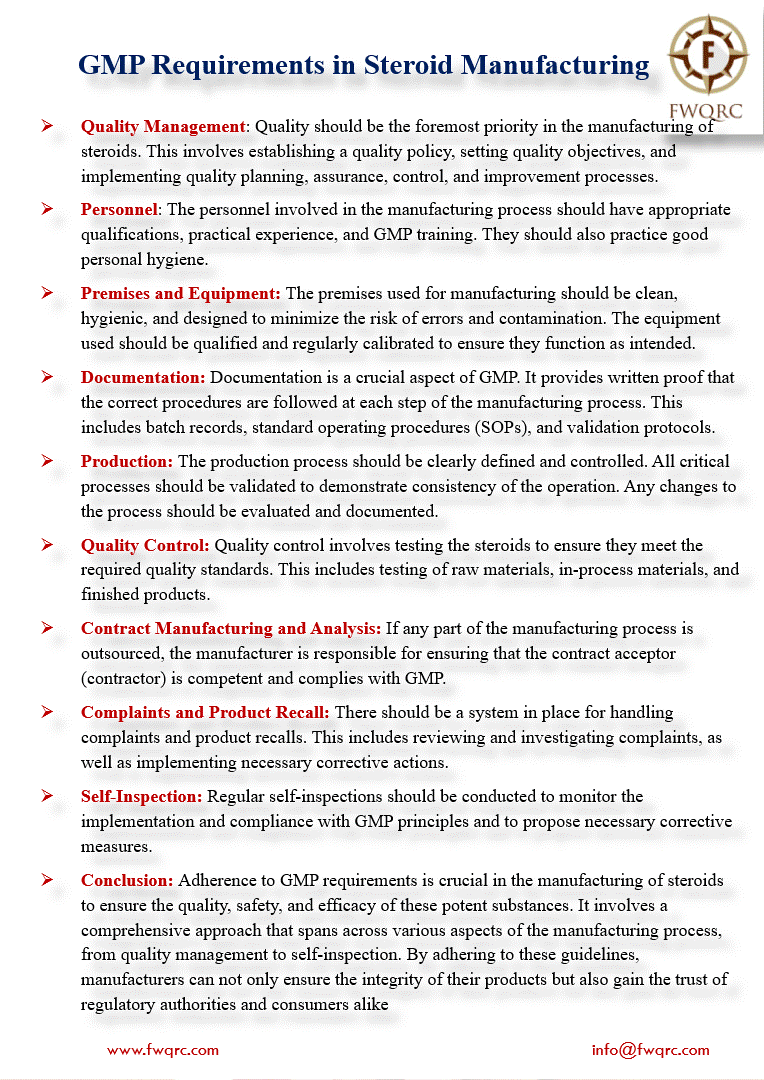
#Hormones and #steroids play a crucial role in #metabolic processes and have a wide range of #pharmaceutical applications, including #contraception, #hormonetherapy, #antirheumatic, #antiarthritic, and #bodybuilding preparations.
The #manufacturing of these substances is #regulated by stringent #guidelines to ensure their #safety and #efficacy. One such set of guidelines is Good Manufacturing Practice (#GMP), which is designed to minimize #risks involved in #pharmaceutical #production. This blog post will delve into the GMP requirements specific to the manufacturing of steroids and ensuring operator health and safety.
The #production of these #drugs are a complex process that requires stringent #safety measures to ensure the #health of #operators and the #quality of the #products.
#Occupational steroid exposure can occur when the #active ingredients are #inhaled, #ingested, or #absorbed through #exposedskin. Although exposure can occur at any point in the production process, the greatest potential for worker exposure during steroid/hormonal drug production occurs during weighing, material transfer, equipment loading, or milling. Unit operations such as #blending, #granulation, #drying, #tablet #compression, and #coating can also present #hazards.
#Manufacturers have the #responsibility to #protect their #operators, particularly those working with #potent and #toxic compounds and set defined areas or #controls necessary to eliminate the #risk of product #crosscontamination on a case-by-case basis.
#Adherence to #GMP requirements is crucial in the manufacturing of steroids to ensure the #quality, #safety, and #efficacy of these #potent substances. It involves a comprehensive approach that spans across various aspects of the manufacturing process, from #qualitymanagement to #selfinspection. By adhering to these guidelines, manufacturers can not only ensure the integrity of their products but also gain the trust of #regulatory #authorities and #consumers alike.
#food #drugs #cosmetics #medicaldevices #medicinalproducts #fda #eugmp #schedulem #cdsco #dca #anvisa #cofepris #tga #ukmhra #edqm #auditor #irca #cqi #auditingservices #consultingservices #qms #qa #qc #qms #qualitymanagementsystem #regulatoryservices #regulatorycompliance #qualitycomplaince #gapanalysis #riskanalysis #capa #remediationplans #trendingposts #trendingnow