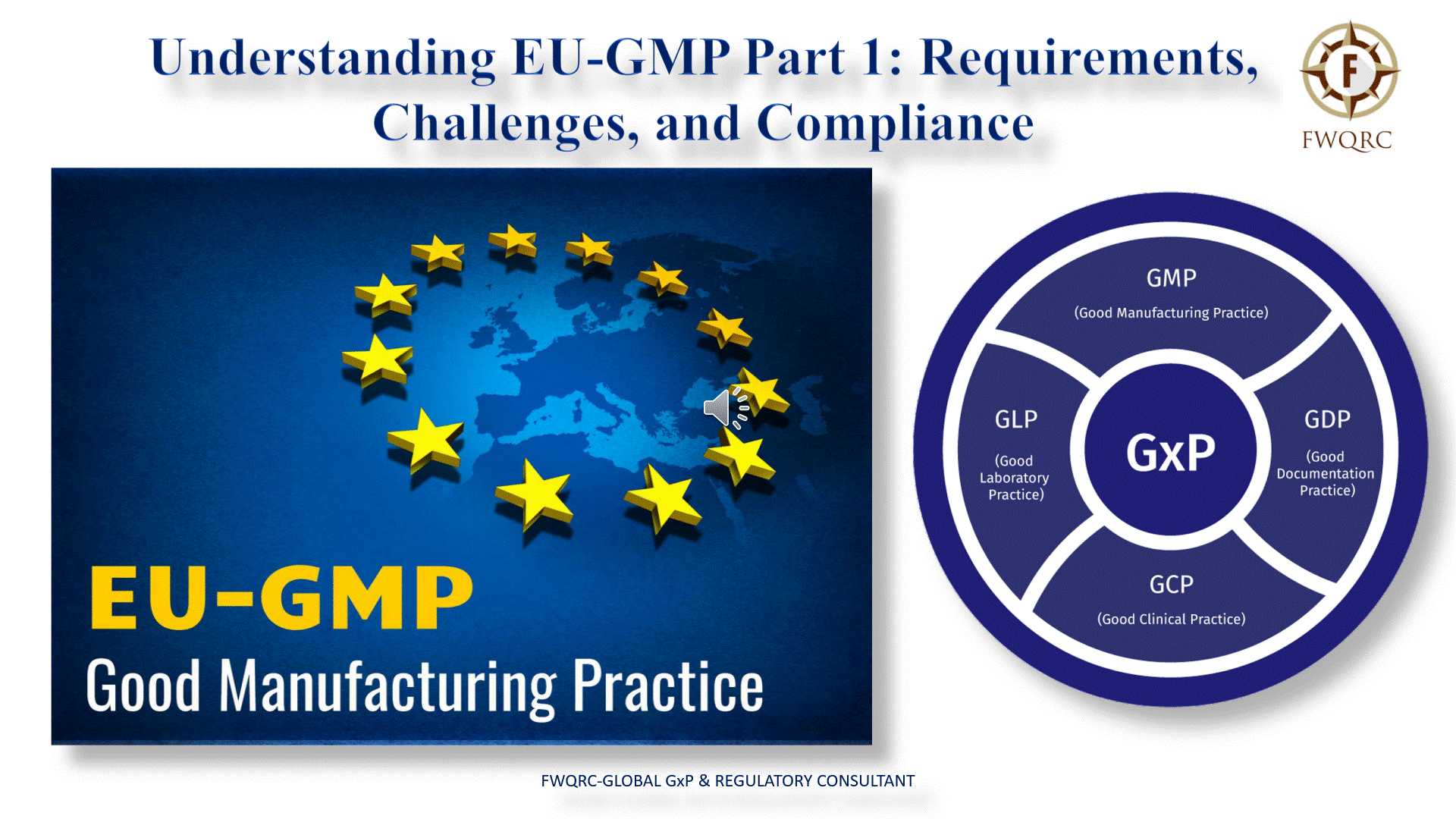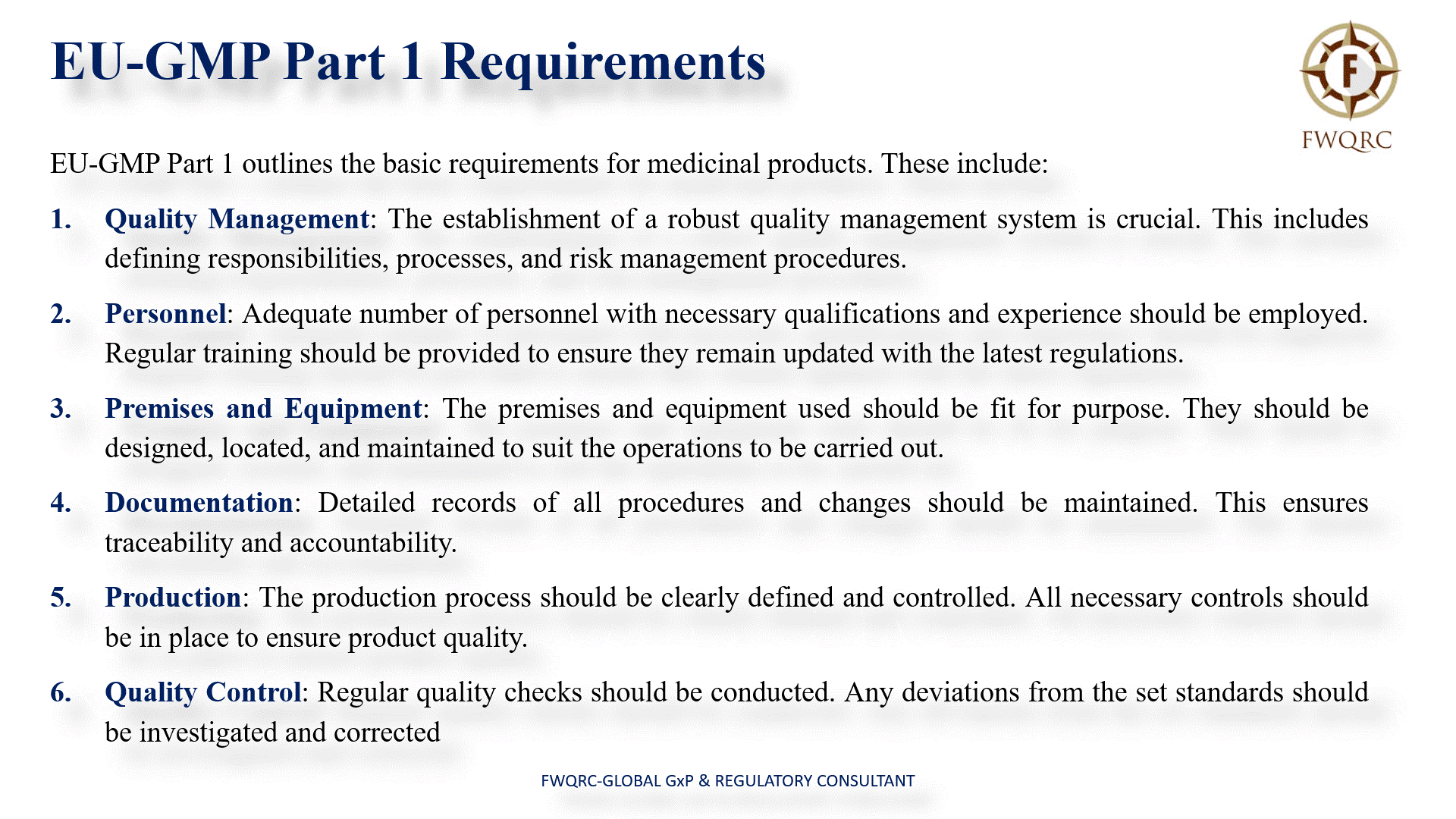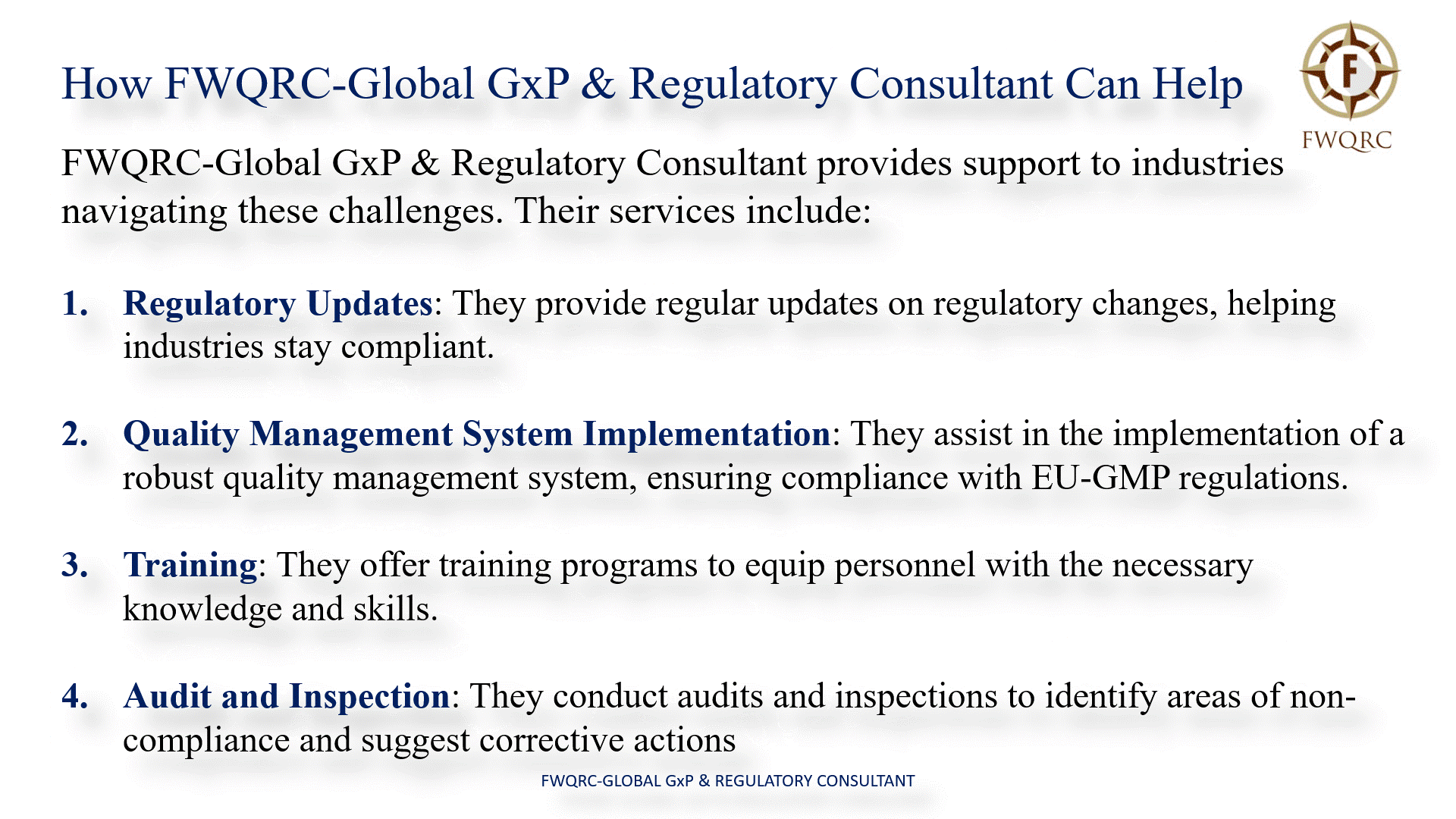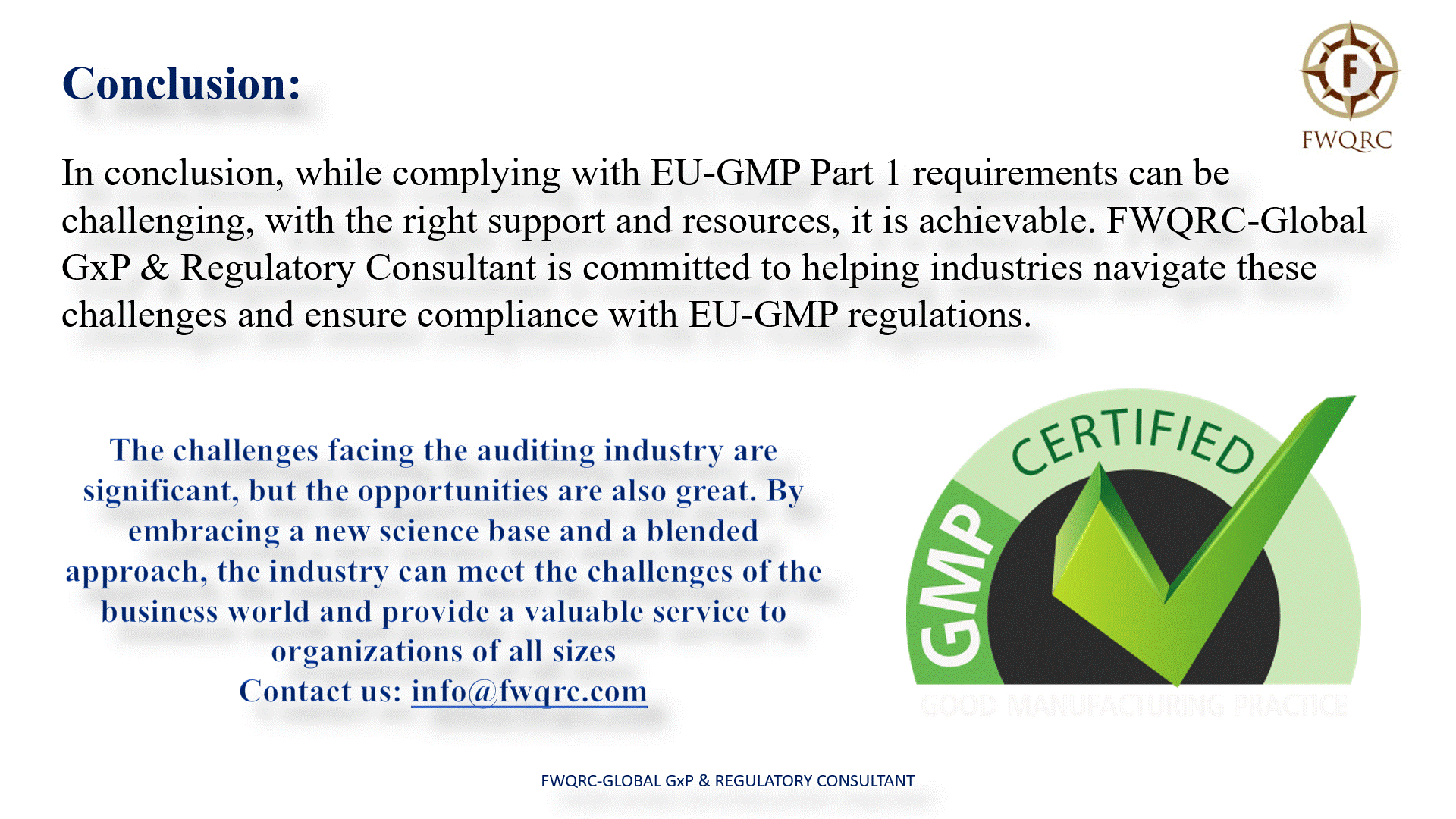
The European Union Good Manufacturing Practice (EUGMP) guidelines are a set of regulations that ensure the consistent production and control of medicinal products. These guidelines are crucial for maintaining product quality and safeguarding public health. In this blog, we will delve into the requirements of EUGMP Part 1, the challenges faced by industries in adhering to these regulations, and how FWQRC-Global GxP & Regulatory Consultant can support industries in ensuring compliance.
EUGMP Part 1 Requirements
EUGMP Part 1 outlines the basic requirements for medicinal products. These include:
- Quality Management: The establishment of a robust quality management system is crucial. This includes defining responsibilities, processes, and risk management procedures.
- Personnel: Adequate number of personnel with necessary qualifications and experience should be employed. Regular training should be provided to ensure they remain updated with the latest regulations.
- Premises and Equipment: The premises and equipment used should be fit for purpose. They should be designed, located, and maintained to suit the operations to be carried out.
- Documentation: Detailed records of all procedures and changes should be maintained. This ensures traceability and accountability.
- Production: The production process should be clearly defined and controlled. All necessary controls should be in place to ensure product quality.
- Quality Control: Regular quality checks should be conducted. Any deviations from the set standards should be investigated and corrected.
Challenges in Compliance
While the EUGMP guidelines are comprehensive, industries often face challenges in adhering to them. These include:
- Keeping Up with Regulatory Changes: The regulatory landscape is constantly evolving, making it challenging for industries to stay updated.
- Resource Constraints: Implementing and maintaining a robust quality management system requires significant resources.
- Technical Challenges: Ensuring that premises and equipment meet the necessary standards can pose technical challenges.
- Training Personnel: Regularly training personnel to ensure they are aware of the latest regulations can be time-consuming and costly.
How FWQRC-Global GxP & Regulatory Consultant Can Help
FWQRC-Global GxP & Regulatory Consultant provides support to industries in navigating these challenges. Their services include:
- Regulatory Updates: They provide regular updates on regulatory changes, helping industries stay compliant.
- Quality Management System Implementation: They assist in the implementation of a robust quality management system, ensuring compliance with EUGMP regulations.
- Training: They offer training programs to equip personnel with the necessary knowledge and skills.
- Audit and Inspection: They conduct audits and inspections to identify areas of non-compliance and suggest corrective actions.
In conclusion, while complying with EUGMP Part 1 requirements can be challenging, with the right support and resources, it is achievable. FWQRC-Global GxP & Regulatory Consultant is committed to helping industries navigate these challenges and ensure compliance with EUGMP regulations.
#food #drugs #cosmetics #medicaldevices #auditing #irca #eugmp