Benefit-risk assessments made to support certain regulatory decisions about NDAs or BLAs, from premarket approval through the postmarket setting. This includes decisions regarding any regulatory requirements for approval, such as inclusion of a boxed warning in approved labeling, postmarketing study requirements and commitments, and risk evaluation and mitigation strategies (REMS). These regulatory decisions are made in accordance with specific, applicable legal and regulatory authorities and criteria. This guidance touches on some of these authorities but does not attempt to list or address them all.
This guidance does not directly address other regulatory decisions that may occur throughout the drug development lifecycle, such as decisions regarding first-in-human trials of an investigational new drug and expanded access applications, which also may require FDA to consider information about the benefits and risks of an investigational or marketed drug for its proposed use. However, the concepts discussed in this guidance may also be relevant to these other types of decisions.
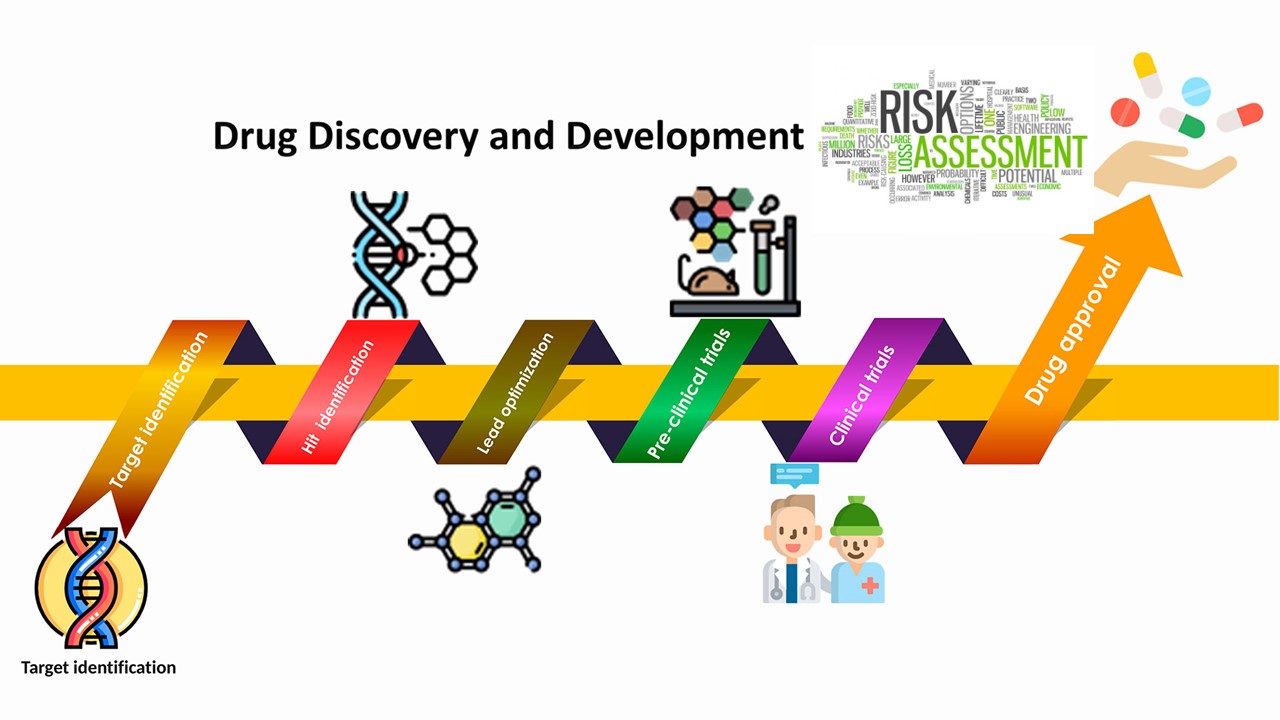
Under the FD&C Act, for a new drug to be approved for marketing in the United States, FDA must determine that the drug is safe and effective for use under the conditions prescribed, recommended, or suggested in the product’s labeling. The demonstration of effectiveness under this standard requires substantial evidence that the drug will have the effect it purports or is represented to have. Because all drugs can have adverse effects, the demonstration of safety requires a showing that the benefits of the drug outweigh its risks.
Benefit-risk assessment is thus integrated into FDA’s regulatory review of marketing applications for new drug and biological products. Broadly speaking, benefit-risk assessment in FDA’s drug regulatory context is making an informed judgment as to whether the benefits (with their uncertainties) of the drug outweigh the risks (with their uncertainties and approaches to managing risks) under the conditions of use described in the approved product labeling.
Takeaway:
The benefit-risk assessment for a new drug can be straightforward in cases when a drug’s benefit is established as clinically meaningful and the drug’s safety profile is well-characterized with no serious risks identified. The benefit-risk assessment becomes more challenging in cases where the potential for serious risks is identified or expected to exist, e.g., risks that are life-threatening or associated with significant morbidity. In cases where serious risks are anticipated, certain findings may nevertheless weigh in favor of a favorable benefit-risk assessment.
Periodic reporting is an important mechanism for sponsors to communicate information to FDA that can inform benefit-risk assessment over the drug’s lifecycle. The ICH guidance for industry E2C(R2) provides recommendations on developing a Periodic Benefit-Risk Evaluation Report (PBRER), which provides an alternative reporting format that may be used in place of the periodic postmarketing safety report described in 21 CFR 314.80(c)(2) and 600.80(c)(2).
If you would like to receive notifications about regulatory guidance, email: info@fwqrc.com