
Cosmetics
FWQRC™ offers one stop solution for cosmetic manufacturers helping them to obtain the manufacturing license
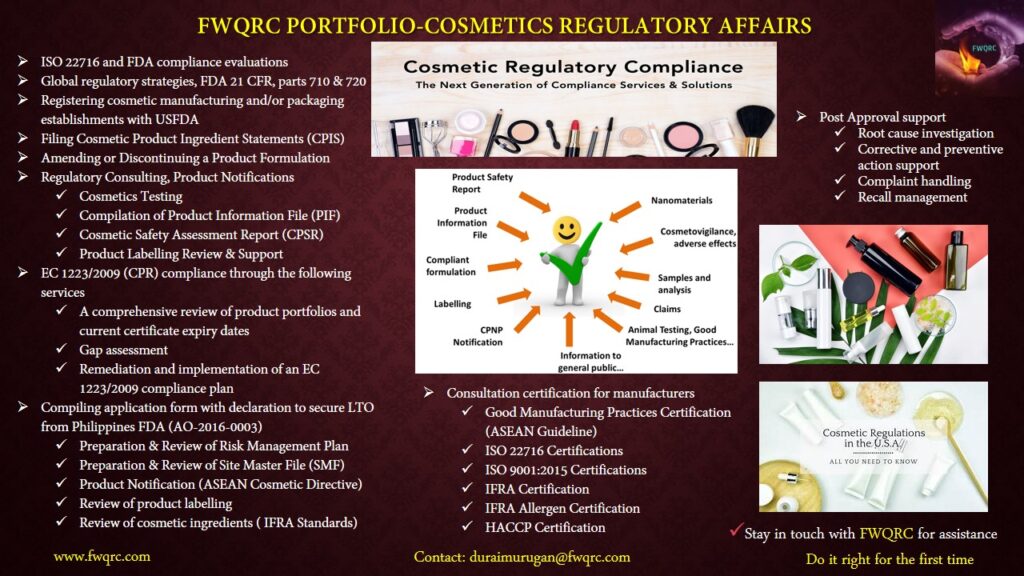
The following license are required to manufacture & market in India according D&C act, 1940 and USFDA
- License in form 32 is issued for manufacturing cosmetics for sale or for distribution. (Application is filed in Form 31)
- License in form 32-A is issued for loan license for manufacturing cosmetics for sale or for distribution. (Application is filed in Form 31-A)
- License on Form 37 is issued for grant or renewal of approval for carrying out tests on drugs/cosmetics or raw material used in the manufacture thereof on behalf of licenses for manufacture for sale of drugs/cosmetics. (Application is filed in Form 36)
- FDA registration and marketing requirements (FD&C Act, sec. 510; 21 CFR 207)
FWQRC™ intended to help companies implement Amendments to the IFRA Code of
Practice, which consists of new or revised Standards
- Standards that impose a quantitative limit on the use of fragrance materials are expressed as a maximum concentration of fragrance material in the consumer product. This implies
knowledge of the concentration of the restricted fragrance material in the compound and the concentration of the compound in the final consumer product. Fragrance suppliers are
therefore required to inform manufacturers of consumer products, who use or intend to use a fragrance compound, that due to the presence of a restricted ingredient, the compound should only be used up to a specified maximum concentration. This can either be a maximum for a number of applications (driven by the most restrictive one) or in the form of an individual listing of maximum concentrations for well-defined applications, thereby being in compliance with IFRA Standards. Unless otherwise specified, concentrations are expressed in weight-perweight percent. - From the 40th Amendment on, the Standards limiting ingredients due to sensitization are based on the Quantitative Risk Assessment for dermal sensitizers (QRA). The QRA methodology for fragrance ingredients is a refined risk assessment approach for dermal sensitizers, which currently identifies individual limitations for 11 specific product categories (based on similar Safety Assessment Factors and exposure). More information on how the dermal sensitization QRA works in detail is available from IFRA or RIFM.
- The QRA methodology as it exists today does not cover occupational use of consumer
products, mainly due to missing exposure data to build into the risk assessment.
Fragrance compounds in medical devices, OTC drugs and topical drugs are not covered by
the current QRA methodology. This is mainly due to the potential or intended application on compromised or diseased skin and a different risk benefit consideration than for typical
consumer products is needed. In addition, these product types are under the scope of specific regulations with defined safety assessment requirements.
- The compliance with the Standards of the IFRA Code of Practice is mandatory for all IFRA member companies belonging to an IFRA member association.
- The IFRA Standards enter into force as follows:
- IFRA Standards of restriction and prohibition:
- 2 months after the date of the letter of notification for new submissions
- 14 months after the date of the letter of notification for existing fragrance compounds
- IFRA Standards with a purity criterion
- 7 months after the date of the letter of notification for new submissions
- 19 months after the date of the letter of notification for existing fragrance compounds
- IFRA Standards of restriction and prohibition:
IFRA recommendations for Good operating practices
- Personnel
- Premises and Sanitation
- Quality Assurance
- Quality Control and Storage
- Manufacturing Operations
- Packaging and labeling
- Distribution
- Health & Environmental protection

