The U.S. Food and Drug Administration (FDA) has recently issued a final rule aimed at enhancing our understanding of antimicrobial use in food-producing animals.
This rule focuses on collecting more detailed information about antimicrobial sales and distribution, specifically by species.
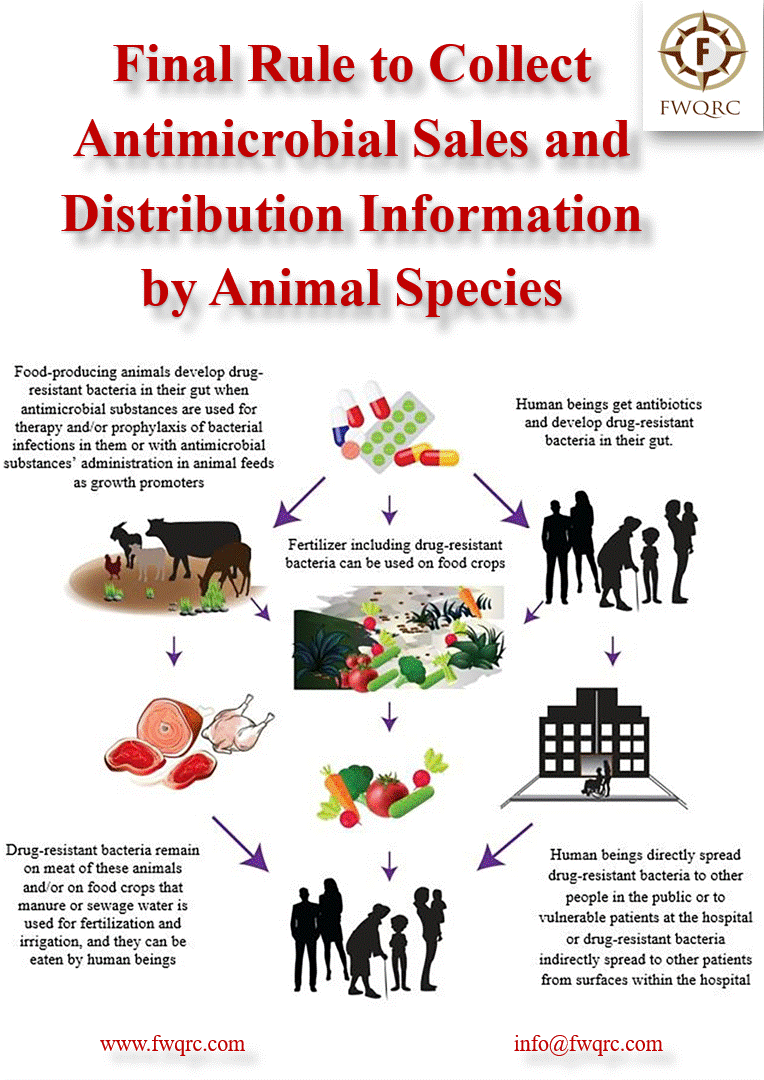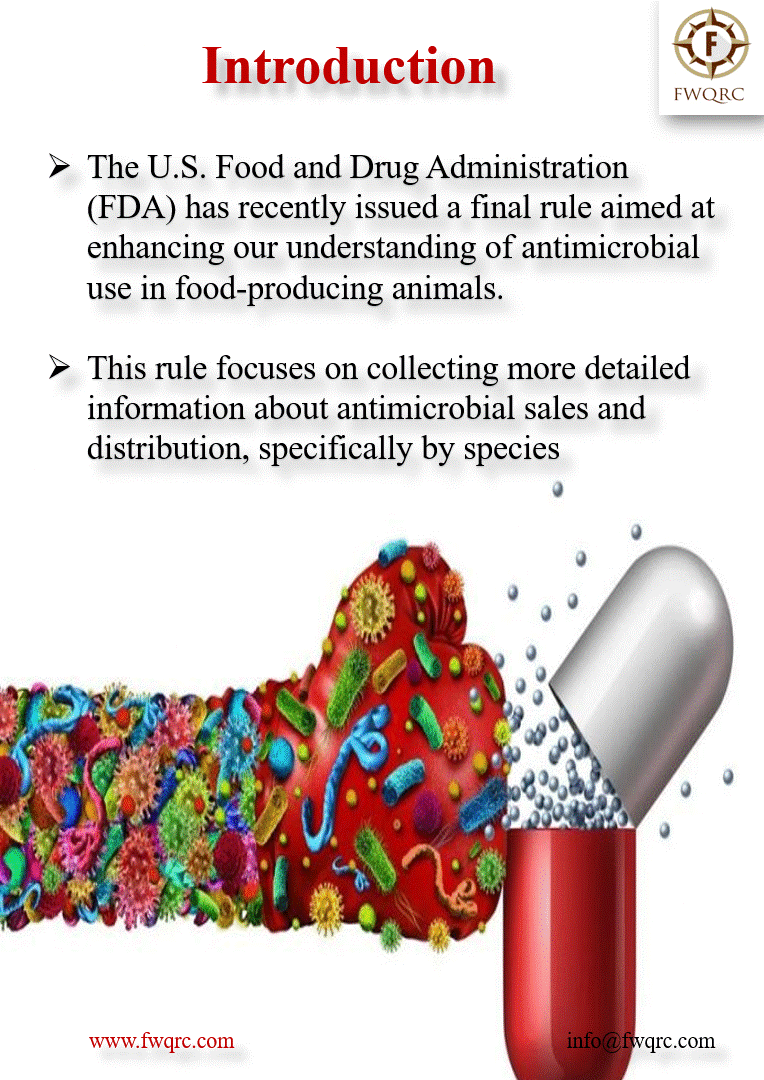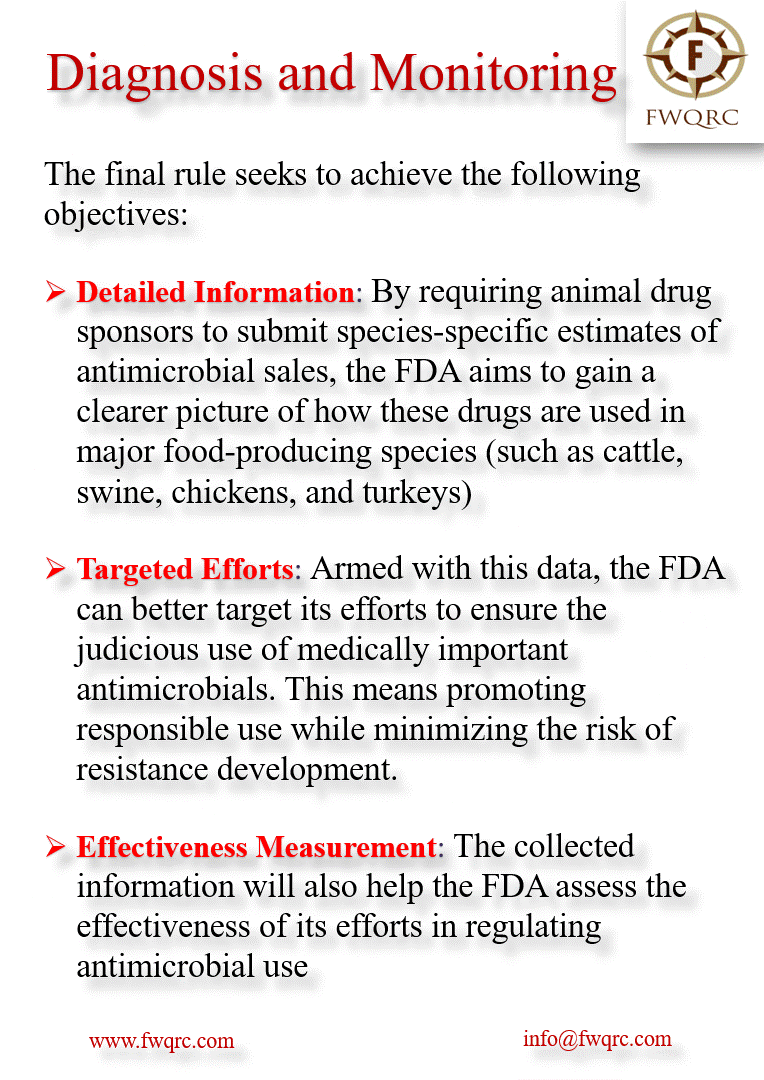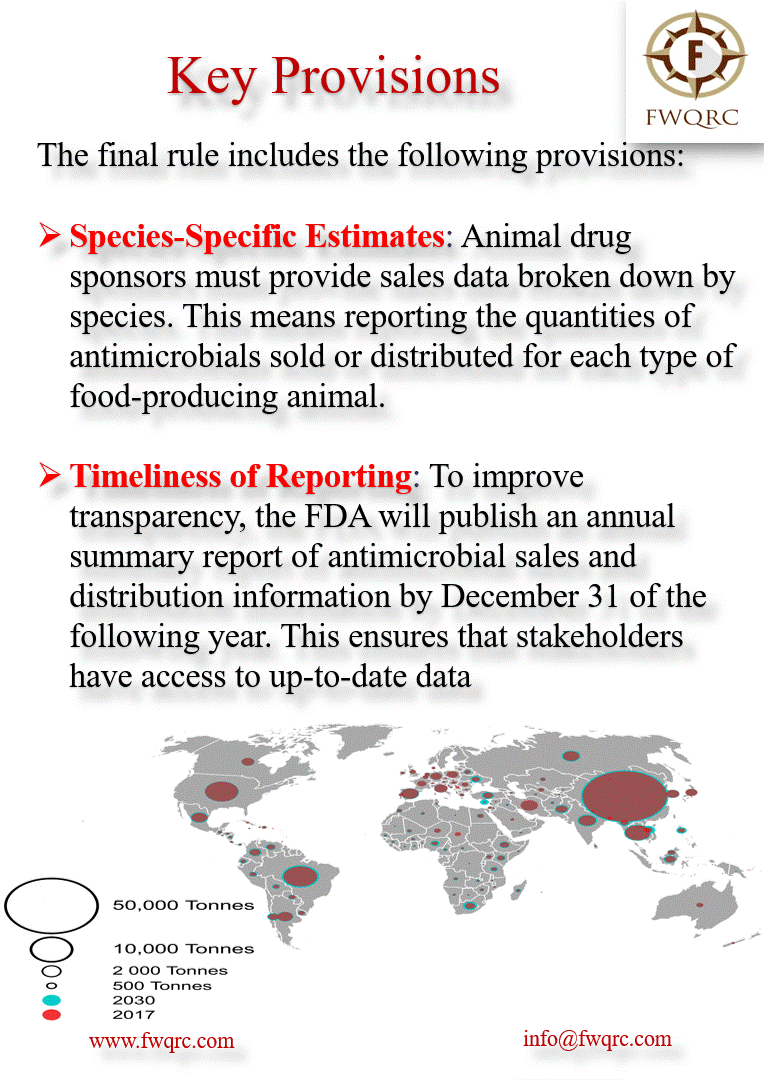
Antimicrobials play a crucial role in veterinary medicine, but their use must be judicious to prevent the development of antimicrobial resistance.
The FDA recognizes the importance of monitoring and regulating antimicrobial use in food-producing animals to safeguard public health.
Diagnosis and Monitoring:
The final rule seeks to achieve the following objectives:
Detailed Information: By requiring animal drug sponsors to submit species-specific estimates of antimicrobial sales, the FDA aims to gain a clearer picture of how these drugs are used in major food-producing species (such as cattle, swine, chickens, and turkeys)
Targeted Efforts: Armed with this data, the FDA can better target its efforts to ensure the judicious use of medically important antimicrobials. This means promoting responsible use while minimizing the risk of resistance development.
Effectiveness Measurement: The collected information will also help the FDA assess the effectiveness of its efforts in regulating antimicrobial use.
The final rule includes the following provisions:
Species-Specific Estimates: Animal drug sponsors must provide sales data broken down by species. This means reporting the quantities of antimicrobials sold or distributed for each type of food-producing animal.
Timeliness of Reporting: To improve transparency, the FDA will publish an annual summary report of antimicrobial sales and distribution information by December 31 of the following year. This ensures that stakeholders have access to up-to-date data.
#antimicrobial #resistance #AMR #animaldrug #gmp #verterinary #auditor #leadauditor #gapanalysis #gapassessment #riskanalysis #capa #qa #qc #qms #qualitycompliance #regulatorycompliance